The first paper regards the development
of a chemical sensor molecule that can sense enantiomeric phenomenons in a
fluorous phase. The compound is a chiral perfluoroalkyl-BINOL based diketone,
and the researchers have shown that this new compound can serve as an
enantioselective fluorescent sensor when it is dissolved into a fluorous phase.
That kind of suggests that, when an enantiomer of this compound encounters a
substrate with a particular enantiomeric configuration, they will interact and
result in a large enhancement in fluorescent signals. The researchers have hypothesized,
and also have confirmed that the rationale of this is due to formation of
large-sized aggregates which can then be observed by dynamic light-scattering techniques.
 |
| The first generation (S)-BINOL. This candidate is not 'fluorous' enough to serve as a fluorous sensor. Modified from [1]. |
Now the big question, how can they make a
greasy BINOL to partition into a fluorous phase? Their first generation
compound will not work, and that illustrates the point of the 2 criteria I have
mentioned above. True, it does contain 2 attention-attracting CF3
groups (which medicinal chemists love because they often positively impact the
activity of a drug molecule), but the compound is simply not fluorous enough.
The fluorine content is way lower than 60%, and they are far away from each
other. So, as they have confirmed, this compound simply dissolves in the
organic solvent and does not crash out as a second phase. So how can they solve
the problem? You get it – increase the fluorine content of the BINOL compound.
So, the second generation BINOL they use in this work, consists of 2 C7F15
groups (a total of 2 sets of 15 closely-packed fluorine atoms here!) and this
is more than enough for the compound to partition into a fluorous phase.
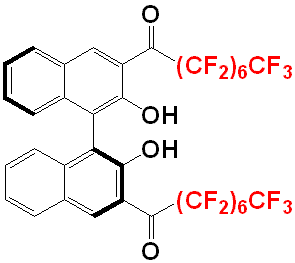 |
| The novel fluorous (S)-BINOL. This compound is fluorous enough to preferentially partitioned into the fluorous phase in a bi-phasic system. Modified from [1]. |
To explain the reaction they study, I will use a notation
and a diagram here. The reaction involves the BINOL and also an amino alcohol,
and both of these compounds are enantiomeric. The 2 enantiomers of the BINOL
are denoted as ‘A’ and ‘a’ (R and S configuration respectively), and the 2 enantiomers
of the amino alcohol are denoted as ‘B’ and ‘b’ (ditto).
 |
| The 'Match' cases that will lead to intense fluorescence signals. Modified from [1]. |
What they have discovered is that the R enantiomer of the
BINOL can interact only with the S enantiomer of the amino alcohol to give a
significant increase in fluorescence signal, and vice versa. That means ‘A’
interacts with ‘b’, and ‘B’ interacts with ‘a’. They have also analyzed the
reaction mixture and are able to propose that a nucleophilic addition has
occurred between the BINOL and the amino alcohol to form an oxazolidine
structure. If A interacts with B (or a interacts with b), the fluorescence
intensity is much smaller – which means a ‘mismatch’ in pair takes place. So,
this is seen as a sort of chiral recognition. The scope of the amino alcohol
can be expanded and even diamines have been tried.
This is certainty a great reaction done in the fluorous
phase, as it involves a highly perfluorinated BINOL compound. Coupled with
fluorescence techniques, that makes the chiral recognition technique easily observable
and quantifiable at the same time.
1. Enantioselective
Fluorescent Recognition in the Fluorous Phase: Enhanced Reactivity and Expanded
Chiral Recognition. C. Wang,
E. Wu, X. Wu, X. Xu,
G. Zhang, L.
Pu.
DOI: 10.1021/ja512569m
